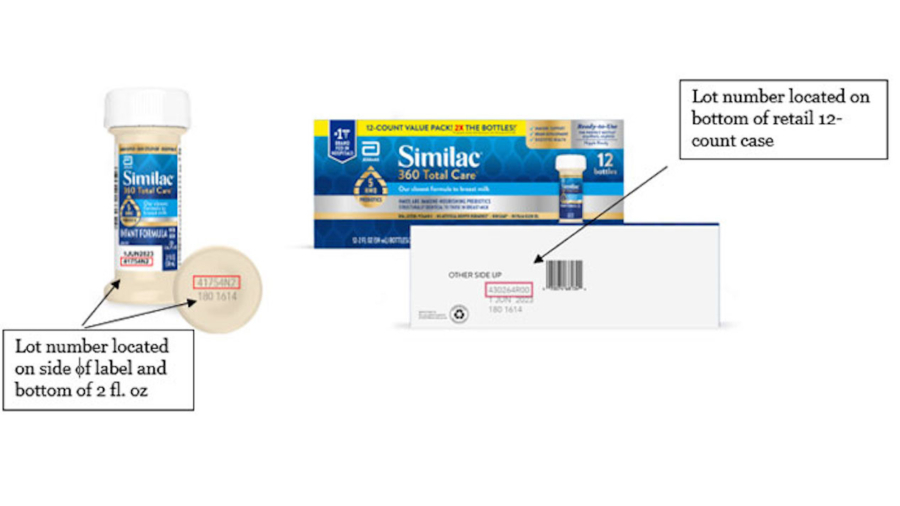
Abbott Nutrition on Friday announced it’s recalling some of its baby formula products sent to hospitals and retailers in the United States, Canada, and the Caribbean because of the risk some may be spoiled.
The company said the voluntary recall is for certain lots of its two fluid-ounce bottles of “Ready-to-Feed” liquid products for infants and children.
The problem is that the caps of some bottles in the recalled lots “may not have been sealed completely,” which the company said could result in spoilage. Spoiled products if consumed could cause gastrointestinal symptoms such as diarrhea and vomiting.
“We take our responsibility to deliver high-quality products very seriously,” Joe Manning, executive VP of nutritional products at Abbott, said in a statement.
“We internally identified the issue, are addressing it, and will work with our customers to minimize inconvenience and get them the products they need.”
The company said the impacted brands include Similac® Pro-Total ComfortTM, Similac® 360 Total Care®, Similac 360 Total Care Sensitive, Similac® Special Care® 24, Similac Stage 1, Similac® NeoSure®, Similac Water (Sterilized), and Pedialyte Electrolyte Solution.
Impacted Products
Less than 1 percent of the bottles in the lot have been identified with this problem, the company said. Other Abbott Nutrition liquid or powder formula brands are not included in the recall, including any amino acid-based formulas or metabolic nutrition formulas.
“This recall equates to less than one day’s worth of the total number of ounces of infant formula fed in the U.S. and is not expected to impact the overall U.S. infant formula supply,” the company said.
Hospitals and health care providers will continue to be able to acquire Similac 2 fluid ounce (59 milliliters) Ready-to-Feed liquid formula products, which the company said are from a different production line.
“Similac infant formula will continue to be produced in alternative product sizes and formats for delivery to retail locations, in addition to increased production throughout our global manufacturing network,” the company said.
The recalled products were mostly distributed to hospitals, some doctor’s offices, distributors, and retailers in the United States, including Puerto Rico.
One lot of products was sent to Barbados, Bermuda, Colombia, the Dominican Republic, Haiti, Jamaica, St. Croix, and St. Thomas. Two other lots were sent to Canada, Curacao, Panama, and Trinidad and Tobago.
Abbott advised parents and caregivers not to use any products identified in the recall. The company provided a detailed list of impacted products and instructions for local contacts in the countries the product was sent to on its website.
Earlier this year, Abbott issued a larger recall for its powdered baby formula products from its Sturgis, Michigan, facility, leading to a nationwide baby formula shortage, after four infants reportedly became ill with a rare infection.
From The Epoch Times