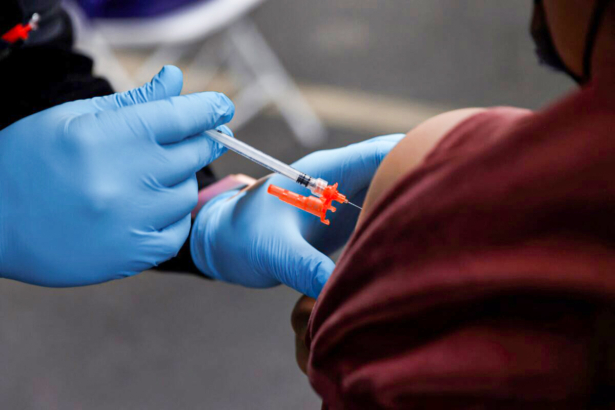
U.S. drug regulators on Dec. 8 announced emergency clearance for two COVID-19 vaccines for children as young as 6 months of age.
The updated vaccines from Pfizer and Moderna are now able to be administered to younger children after previously only being available to those 5 and older, the U.S. Food and Drug Administration (FDA) said.
The old vaccines are based on the Wuhan strain’s spike protein. The new vaccines contain components of both the Wuhan strain and the BA.4/BA.5 subvariants. They’re known as bivalent shots.
In most ages, the new vaccines can only be used as boosters. That’s also the case for young people who take a Moderna primary series, which is two doses. But because Pfizer’s primary series for young children is three shots, the new jab is authorized as a third dose.
Justifying their decision, officials baselessly suggested the vaccines protect against severe illness and hospitalization.
“As this virus has changed, and immunity from previous COVID-19 vaccination wanes, the more people who keep up to date on COVID-19 vaccinations, the more benefit there will be for individuals, families, and public health by helping prevent severe illnesses, hospitalizations, and deaths,” Dr. Robert Califf, the FDA’s commissioner, said in a statement.
There is no clinical efficacy data for the updated shots. Regulators relied on data from mice and from a different set of updated vaccines when initially authorizing them in the fall, and said safety data was from the old vaccines.
“How can any doctor look at parents and insist this is ‘safe’ for their babies when it was never tested on people?” Kim Witczak, a drug safety advocate, wrote on Twitter.
The only effectiveness estimates, drawn from real-world settings by the U.S. Centers for Disease Control and Protection, found that protection against symptomatic disease was subpar, particularly for younger people.
And multiple studies, measuring the response triggered by the updated vaccines, found they perform poorly against the rising variants in circulation, including BQ1.1.
Dr. Harvey Risch, a professor emeritus of epidemiology at the Yale School of Public Health, told The Epoch Times this week that the original vaccines, much less the largely untested new ones, should not have been authorized for young children in the first place.
He noted that BA.4 and BA.5 have either disappeared from the United States or are on the downslope while newer variants are becoming dominant.
Letters
In their letters to the vaccine manufacturers amending emergency use authorizations for the bivalents, regulators were more cautious with their language.
Based on the current evidence, “it is reasonable to believe” that the vaccines “may be effective,” they wrote. They also said that it is “reasonable to conclude, based on the totality of the scientific evidence available, that the known and potential benefits” of the vaccines “outweigh the known and potential risks.”
The authorizations are for the prevention of COVID-19. Risks include side effects like heart inflammation and Bell’s Palsy.
The FDA, continuing a growing trend, did not consult with its vaccine advisory panel before issuing the new authorizations.
Both Moderna and Pfizer celebrated the new development.
“With the FDA’s decision, children and adolescents of all age groups in the U.S. will now be eligible for our updated bivalent COVID-19 booster, providing families with an important protective tool as we continue through the winter months,” Stéphane Bancel, Moderna’s CEO, said in a statement. “We appreciate the FDA’s timely review.”
“This authorization offers an opportunity for parents to help better protect their young children against COVID-19, including disease caused by Omicron sublineages,” added Albert Bourla, Pfizer’s CEO.
Next Steps
The U.S. Centers for Disease Control and Prevention (CDC) must decide whether all children in the youngest age group should get the updated vaccines. The CDC has never diverged from the FDA during the pandemic.
Pediatricians and others have already been able to preorder the bivalents, enabling them to be shipped soon.
“There will be a sufficient but finite supply of pediatric bivalent COVID-19 vaccines for these age groups, which should be directed to providers with expected demand among eligible patients,” the CDC said in an operational planning guide.
The projected demand isn’t clear, as many parents have opted against getting their babies and toddlers vaccinated.
Just 1.8 million children under 5 have received one or more doses of a vaccine as of Nov. 30. According to U.S. census data, nearly 19 million children under 5 reside in the United States.
The FDA said it would consider authorizing another dose of the new vaccine as a booster for children who receive Pfizer.
“The data to support giving an updated bivalent booster dose for these children are expected in January,” the FDA said. “The agency is committed to evaluating those data as quickly as possible.”
From The Epoch Times


