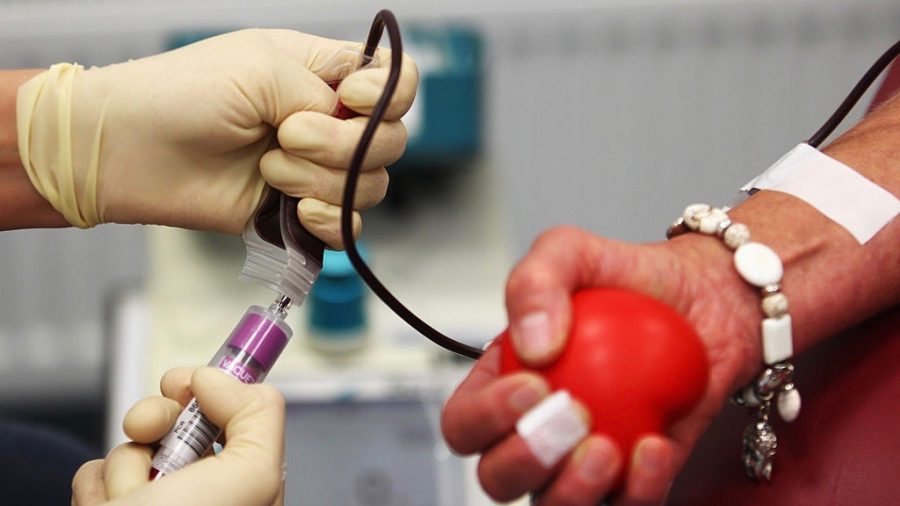
The Food and Drug Administration (FDA) issued a statement on Friday, April 3, regarding an investigational new drug (IND), which uses convalescent plasma, as a response to the CCP Virus
During a Task Force Briefing regarding the CCP (Chinese Communist Party) virus, commonly known as novel coronavirus, Stephen Hahn, the commissioner of the FDA stated that the administration had set up a plasma program where patients who had recovered from the CCP Virus could donate their plasma to help other patients.
Hahn explained during the briefing that the convalescent plasma program involves taking plasma from recovered patients and transferring it into currently infected patients, hopefully transferring immunity to help them recover.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” according to the statement issued by the FDA.
“This is a situation where someone who’s recovered from the virus and doesn’t have the virus in their system at all, you can take plasma…and that plasma contains the proteins in the blood that have the antibodies against the virus. You can take that, process it, and then give it to someone who’s ill. And so that allows you to transfer that immunity,” Hahn said further.
He also said only those eligible can donate and the required testing protocols must be followed. The donation must be found suitable and it can also be donated on a regular basis.
“We’re hopefully expanding that across the country. The Red Cross is involved in that program. And I think it shows a great promise,” Hahn said. He also mentioned that although it does show promise, it still requires more studies into the process.
The Epoch Times reported that on March 27, the first U.S. patient received the experimental convalescent plasma treatment in a hospital in Texas. On March 28, a second patient received the same treatment.
In addition, convalescent plasma has been shown to benefit some patients with the CCP Virus in other countries, and so, the administration is enthusiastic about the process, according to the briefing.
The FDA stated that this process had been studied previously during other respiratory infection outbreaks, such as the 2009–2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic.
Hahn indicated during the briefing, that the process still needs to be studied through clinical trials. Currently, convalescent plasma doesn’t show any indication that it’s effective. When it is proven to be effective and safe through trials, it will then be administered to actual infected patients.
There are various ways to access the treatment immediately, including clinical trials, expanded access, and single-patient emergency IND but protocols must be followed.