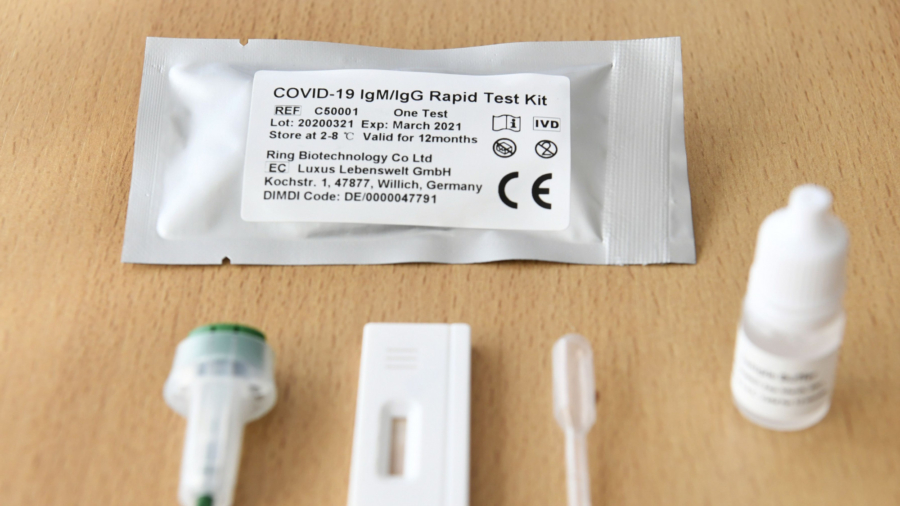
An order prohibiting self-testing for COVID-19 using so-called rapid pinprick tests has been issued in South Australia.
The serology antibody tests can return results in about 30 minutes, but are only accurate if antibodies can be detected in the blood, which can take between five and seven days after infection.
The Therapeutic Goods Administration says because antibody tests do not detect active viral shedding, they cannot determine if an individual is infectious.
That could result in false-negative results.

South Australian police say a direction (pdf) under the Emergency Management Act has been issued to prevent the use of serology tests as a tool to detect or diagnose COVID-19.
“The purpose of this direction is to prohibit a person from using a serological test as an acute illness diagnostic tool for COVID-19, as their use may adversely affect the prevention, control, and abatement of the serious public health risk,” a statement said.
The direction does not apply to employees of South Australia Pathology or the Department of Health and Wellbeing.
Members of the public have also been warned against purchasing test-at-home kits being advertised on the social media platform WeChat in Australia, the ABC reports.
The sale of self-testing kits is illegal in Australia.
“The supply of self-tests or at-home tests for most serious infectious diseases, including self-tests for COVID-19, is prohibited under the Therapeutic Goods (Excluded Purposes) Specification 2010,” a Department of Health spokesperson told the ABC.
“Non-compliance with the regulatory scheme is being monitored and we are working closely with the Australian Border Force and other health and law enforcement agencies.”
“The TGA (Therapeutic Goods Administration) has and will continue to encourage reporting of potential non-compliance via the TGA website for investigation and action.”
The sale of unapproved items can result in up to five years’ imprisonment and A$840,000 in fines per individual. Businesses face steeper fines of up to A$4.2 million.
NTD staff contributed to this report.